Precious metal catalysts are widely used in important chemical fields such as energy, environmental protection, and food processing. How to improve the utilization rate of precious metals while maintaining high catalyst activity, selectivity and long service life has always been a core issue in the development of precious metal catalysts. Recently, Prof. Zheng Nanfeng from Xiamen University has made important progress in the research on the preparation, characterization and catalytic mechanism of platinum nanocomposites. Related research results (“Interfacial Effects in Iron-Nickel Hydroxide–Platinum Nanoparticles Enhance Catalytic Oxidation†") published in the journal Science.
For supported noble metal catalysts, there is a subtle metal-oxide interfacial synergistic effect between the oxide support and the metal nanoparticles, so the performance of the different oxide-supported metal nanoparticles in the catalytic reaction is quite different. The practical application of the catalyst system is often too complicated, it is difficult to analyze the fine structure of the relevant catalytic interface by the existing characterization techniques, which hinders the researchers' in-depth study of the mechanism of action of the catalyst and the relationship between the structure and effect of the reaction. Professor Zheng Nanfeng's research group skillfully applied the wet chemical method to prepare a model nanocatalyst that facilitates the study of the interface effect of precious metal-oxide. Through close collaboration with various research groups at home and abroad, and advanced research methods and theoretical simulations, The Pt-FeNi(OH)x interface synergistically promotes the mechanism of CO catalytic oxidation. Based on their in-depth understanding of the catalytic mechanism, they further developed a more practical Pt-based catalyst preparation method, which makes the reactive interface develop from one-dimensional to three-dimensional of traditional catalysts, and the ratio of active sites to total platinum atoms can reach 50% or more. . The developed catalyst not only can realize the catalytic oxidation of carbon monoxide at room temperature, but also can catalyze the selective oxidation of carbon monoxide under hydrogen-rich conditions and the removal of a small amount of hydrogen under oxygen enrichment. The catalytic lifetime can be as long as more than one month.
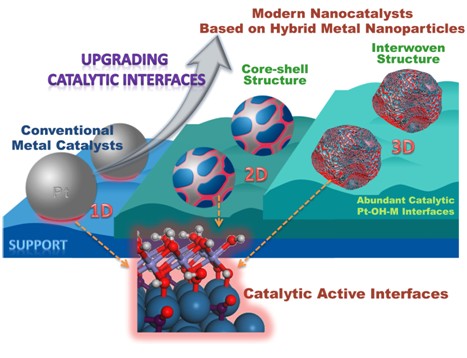
Based on the solid foundation and accumulated experience of Zheng Nanfeng's research group in the controllable synthesis and catalytic performance of precious metal nanocrystals for many years, they first deposited sub-monolayer iron hydroxide on the surface of small and uniform platinum nanocrystals. III), the Fe3+-OH-Pt interface was successfully constructed on the surface of the prepared Pt/Fe(OH)x core-shell composite nanoparticles, combined with sub-Earth spherical aberration-corrected high-resolution transmission electron microscopy and synchrotron radiation X-ray Advanced characterization methods such as absorption spectroscopy and high-sensitivity low-energy ion scattering spectroscopy are used to analyze the fine structure of the Fe3+-OH-Pt interface. Compared with the conventional Pt nanoparticle catalyst, the activity of the Pt/Fe(OH)x composite nanoparticle catalyst in catalyzing CO oxidation is significantly improved. On this basis, in cooperation with Associate Professor Fu Gang, the mechanism of promoting the catalysis of Fe3+-OH-Pt interface was studied by density functional theory. It was found that once CO is adsorbed on the interface Pt, it can be adjacent to OH. Coupling occurs and CO2 is formed after rapid dehydrogenation, indicating that OH at the interface is an active species that oxidizes CO. After CO2 desorption, a low-cost Fe with coordination unsaturation is formed at the interface. These Fe sites are easy to adsorb and activate O2. The activated oxygen species can be oxidized and adsorbed to CO molecules adjacent to the Pt site, and assisted by water vapor. The original Fe3+-OH-Pt active interface is restored, so that the process can be continuously circulated. The proposed mechanism of CO+OH was confirmed by catalytic kinetics and isotope labeling experiments, confirming the importance of interfacial hydrogen-oxygen species in catalysis. It has also been observed experimentally that the Fe3+-OH-Pt interface is prone to water loss during the reaction, resulting in catalyst deactivation, and this problem can be solved by introducing Ni2+. It is found that Ni2+ can form a stable hydrotalcite-like structure together with Fe3+, which stabilizes the Fe3+-OH-Pt interface and greatly improves the life of the catalyst.
On the basis of deep understanding of the synergistic effect of Pt-FeNi(OH)x interface, Zheng Nanfeng's research group further proposed an alloy-assisted synthesis strategy to facilitate the preparation of more practical high-activity, high-stability noble metal nanocatalysts. . The strategy includes the following key steps: firstly preparing PtFeNi alloy nanoparticles by high-temperature reduction of mixed Pt, Fe, Ni precursors in a reducing atmosphere; then, the alloy nanoparticles are naturally oxidized in air to synthesize Pt-FeNi with interwoven structure ( OH)x composite nanoparticle catalyst. The synthesized nano-catalyst has uneven surface, Pt and Fe3+(Ni2+)OHx are intertwined in three dimensions, and have richer active Fe3+-OH-Pt interface, which greatly improves the utilization rate of Pt and significantly reduces the utilization rate. The cost of the catalyst. Studies have shown that the Pt utilization rate of this new type of catalyst is 1.4-1.8 times higher than that of core-shell Pt/FeNi(OH)x nanoparticles, and it can achieve 100% conversion of CO at room temperature, and it will not decay for 1 month. . The catalyst developed can also be used for the selective oxidation of CO under hydrogen-hydrogen conditions and the removal of a small amount of H2 under oxygen-rich conditions.
The research work was carried out under the leadership of Professor Zheng Nanfeng, and was jointly worked by various research groups at home and abroad and at home and abroad. It took three years to complete. Zheng Nanfeng, Fu Gang, Chen Mingshu and other three research groups work closely together to be responsible for the synthesis, characterization, performance testing and catalytic mechanism of the catalyst. Researcher Gu Lin of the Institute of Physics, Chinese Academy of Sciences is mainly responsible for the sub-Earth spherical aberration correction of high resolution transmission of nanoparticles. Electron microscopy studies; Professor Zhang Peng from the Department of Chemistry of Dalhousie University in Canada and Researcher Li Zhiwei from the Center for Synchrotron Radiation Research in Taiwan participated in the simultaneous radiation X-ray absorption spectroscopy study of the catalyst. It is worth mentioning that the first author of the research work (Chen Guangxu) and the second author (Zhao Yun) are doctoral students of Xiamen University.
The work was approved by the National Natural Science Foundation of China (Project Approval Number, 20925103, 21373167, 21033006, 21333008), Ministry of Science and Technology (Project Approval Number: 2011CB932403), Xiamen University, State Key Laboratory of Physical Chemistry of Solid Surfaces, Collaborative Innovation of Energy Materials Chemistry Funding and support from the Center and the National Engineering Laboratory for Alcohol Ether Chemical Clean Production.
A Silver Mirror is a type of glass mirror. Silver mirrors are commonly known as waterproof mirrors, mercury mirrors, silver-plated mirrors on glass surfaces, glass mirrors, mirror glass, etc. Silver mirrors are widely used in furniture, handicrafts, decoration, bathroom mirrors, cosmetic mirrors, optical mirrors and car rearview mirrors. When storing mirrors, they should not be stacked with alkaline and acidic substances, and should not be stored in a humid environment.
In addition, we also sell Silver Mirror glass, silver mirror commonly known as waterproof mirror, mercury mirror, silver-plated mirror on glass surface, glass mirror, mirror glass, etc. Silver mirrors are widely used in furniture, handicrafts, decoration, bathroom mirrors, cosmetic mirrors, optical mirrors, and car rearview mirrors.

Clear Silver Mirror,Clear Silver Mirror Bar,Clear Silver Mirror Bathroom,Clear Silver Mirror Lens
Dongguan Huahui Glass Manufacturing Co.,Ltd , https://www.antiquemirrorsupplier.com